Our Science
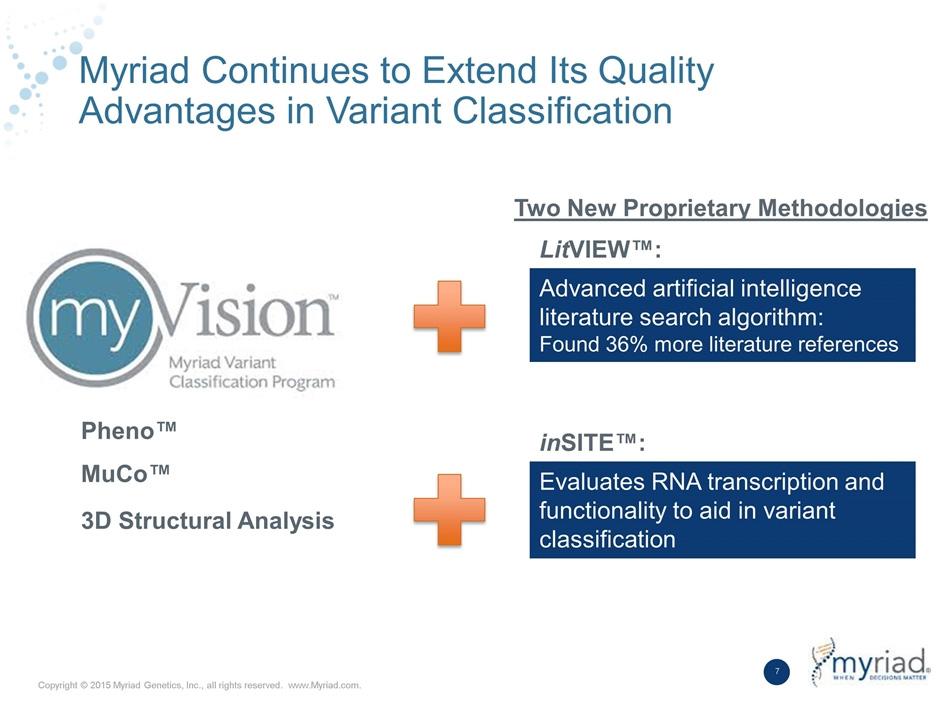
myVision® – Myriad Variants Classification Program
Why is Variant Reclassification in Hereditary Cancer Testing Important?
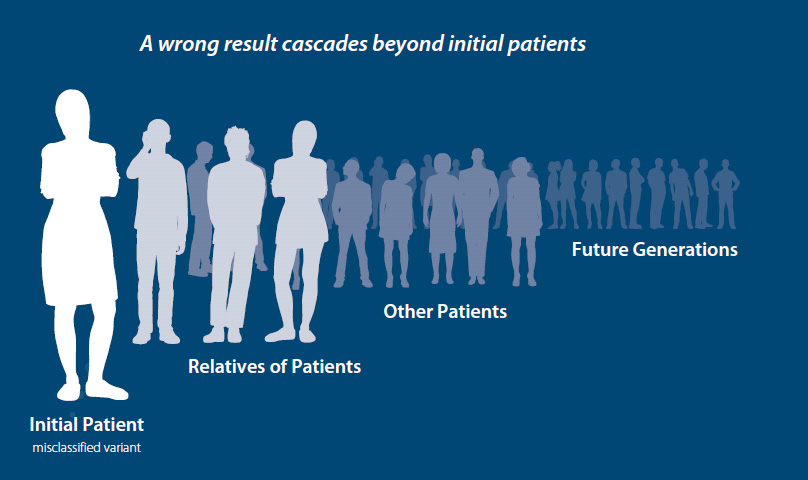
Variant classification is a key component of germline testing, allowing patients to have an accurate answer when they receive a result and stay informed as new information leads to reclassification. Not all labs send amended reports or have variant classification programs, leading to a gap in patient care. Having a robust variant classification program with several tools to classify and reclassify variants is vital to providing the highest level of patient care.
myVision® – Myriad Variants Classification Program
Variant classification is a key component of germline testing, allowing patients to have an accurate answer when they receive a result and stay informed as new information leads to reclassification. Not all labs send amended reports or have variant classification programs, leading to a gap in patient care. Having a robust variant classification program with several tools to classify and reclassify variants is vital to providing the highest level of patient care.
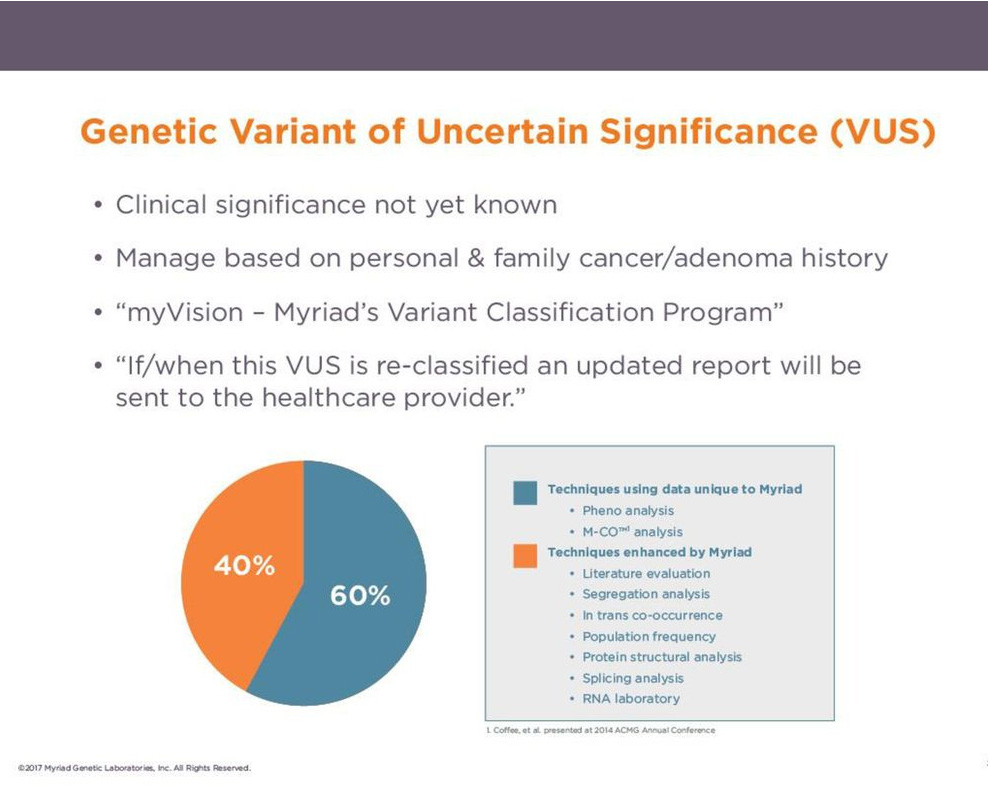
Myriad’s Classification: Variant of Uncertain Significance (VUS)
We recognize that results are used for life-changing decisions, so we prioritize accuracy over speed to classifications:
-Myriad requires a 99% confidence threshold for classifications
-Multiple lines of evidence required for classifications
– Lifetime reclassification commitment: amended

myVision®: Myriad’s unmatched Variant Classification Program
myVision® incorporates several proprietary, clinically validated tools as well as other advanced methods to provide the most comprehensive variant classification program available.
Enhanced Methods by Myriad
• Literature Review: Myriad has an automated literature search engine and employs dedicated PHD level scientists to review the literature1
• Population Analysis and Segregation Analysis: Myriad’s optimized approach as described in Eggington, 20132
• In Trans Co-Occurrence & Homozygosity: Demonstrated to be >99% accurate in Fernandes, 2015
• Structural Biology Analysis: Myriad’s approach described in Kerr, 20164
• Functional RNA Splice Site Analysis: Myriad developed the first research laboratory dedicated to RNA analysis, the approach of which is described in Warf, 20155
Advanced Methods Unique to Myriad
- M-CO™: Myriad’s unique mutation co-occurrence statistical model was developed and validated with >400,000 patients and demonstrated to be >99% accurate in Coffee, 20156
- Pheno™: Myriad’s unique family history weighting tool developed and validated for BRCA 1/2, MLH1, MSH2, MSH6, ATM, CHEK2, PALB2, and BARD1. Demonstrated to be 99% accurate in multiple publications7-9
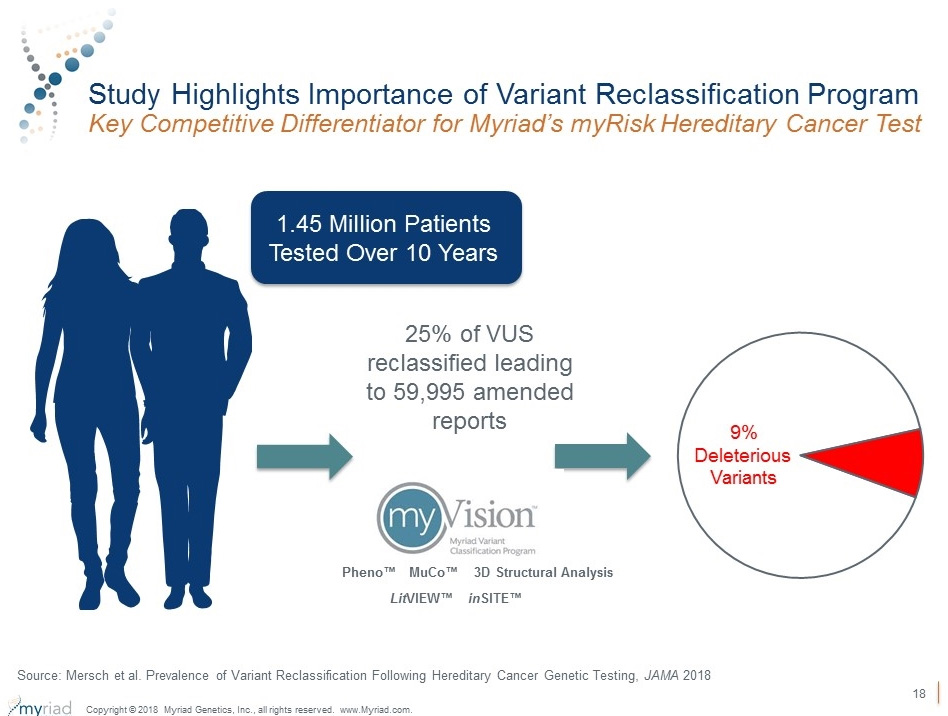
myVision® is a lifetime commitment to patients, with review of variants and updated amended reports sent out to patients daily10
- 60,000+ amended reports between 2006 and 2016
- 2,868 variants reclassified between 2006 and 2016
- 9% of these reclassifications impacted recommended medical management
Myriad’s myVision™Variant Classification program is considered the gold standard in the industry
- We employ over 30 medical professionals of varying disciplines who meet daily to classify and reclassify variants
- We use 8 published and peer-reviewed methodologies, 2 unique to Myriad that have >99% PPV or NPV (11,12,13)
- We classified over 10,000 variants in 2016, and sent over 400 reclassified results to providers every week
- Lifetime commitment to classification: We will provide you and your patients an updated result if a variant is reclassified, no matter when the original testing occurred
Myriad has a diverse team of experts involved in variant classification, with review occurring daily.
References:
- Esterling, L, et al. ASHG 2015.
- Eggington J, et al. Clinical Genetics 2013.
- Fernandes P, et al. ACMG 2015.
- Kerr I, et al. International Symposium on HBOC 2016.
- Warf B, et al. International Symposium on HBOC 2016.
- Coffee B, et al. ACMG 2015.
- Pruss D, et al. Breast Cancer Research and Treatment 2013.
- Morris B, et al. BMC Genetics 2016.
- Bowles K, et al. ACMG 2016.
- Mersch J, et al. JAMA 2018.
- Pruss D et al. Development and validations of a new algorithm for the reclassification of genetic variants identified in the BRCA1 and BRCA2 genes; Breast Cancer Research and Treatment. 2013
- Morris B et al. Classification of genetic variants in genes associated with Lynch syndrome using a clinical history weighting algorithm; BMC Genetics. 2016
- Bowles K et al. Reclassification of uncertain variants in high and moderate cancer risk genes using history weighting analysis; ACMG. 2016
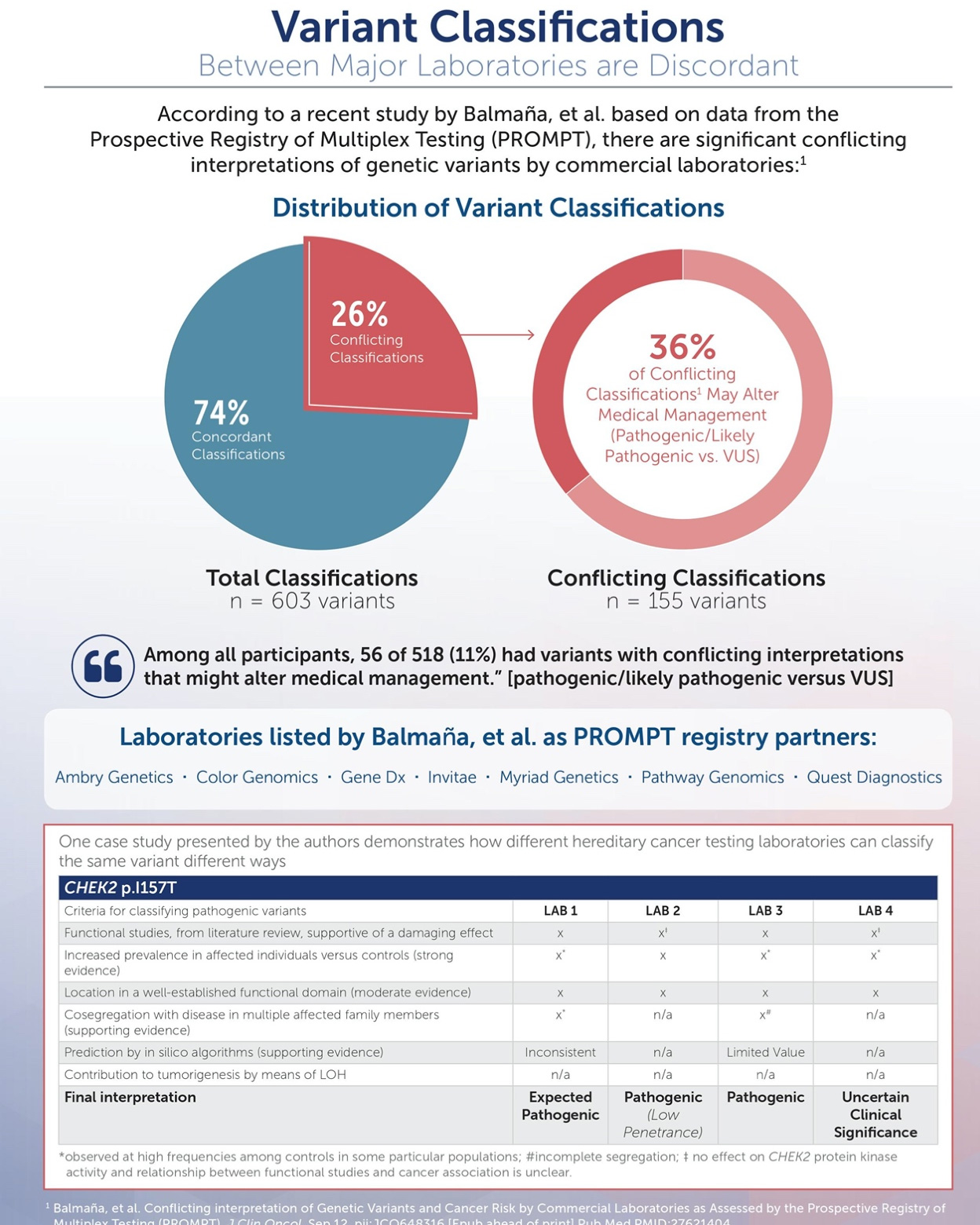
Laboratories Approaches Vary

“As commercially available tests differ in the specific genes analyzed (as well as classification of variants and many other factors), choosing the specific laboratory and test panel is important.”
– NCCN Guidelines Version 1.2016 Genetic/Familial High-Risk Assessment

“The processes by which different laboratories assure the analytic and clinical validity and clinical utility of the sequence variants identified by their NGS analyses may vary… variable methods of interpretation and reporting of the clinical significance and actionability of variants could lead to compromises in patient care.”1
- Robson, et al. Journal of Clinical Oncology 2015

ACMG Guidelines are NOT a definitive variant classification program, and “following” the guidelines does not quantify a lab’s interpretive accuracy.1
- Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015 May;17(5):405-24.
Public databases and less accurate techniques, such as in silico prediction models like SIFT and PolyPhen, are NOT used as part of the Myriad variant classification program due to published data demonstrating their inaccuracy.
Classifications differ between laboratories
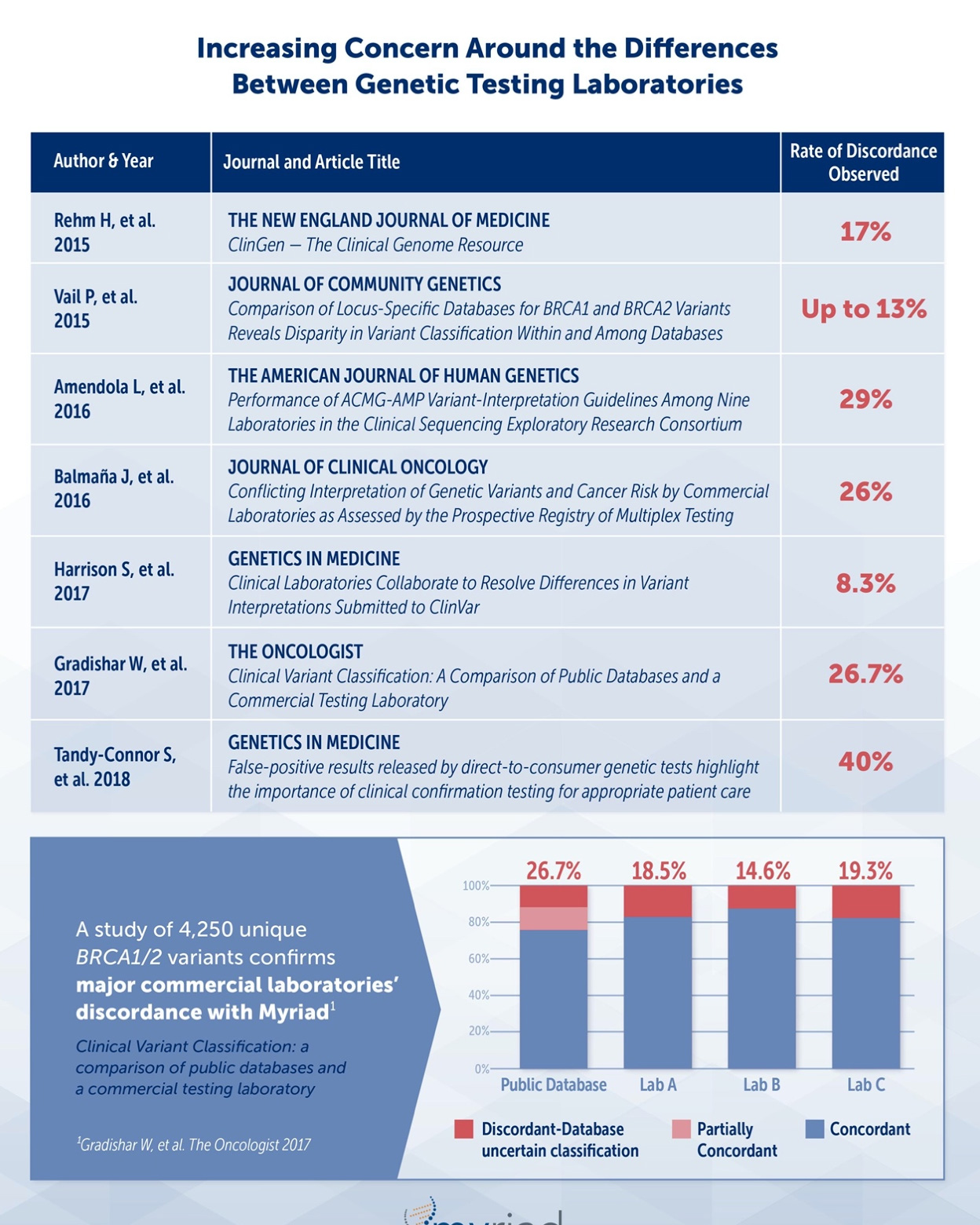
Not only are professional organizations recognizing the discordance between laboratories but multiple peer-reviewed publications have documented a wide range (8.3%-40%) of variance that could impact critical decision making for appropriate patient care.